Go To
- Voltaren Emulgel Extra Strength Diclofenac Diethylamine 2.32% w/w
- Low systemic absorption
- Supported by the National Institute for Health and Care Excellence Clinical Knowledge Summary for Mild-Moderate Pain
- For patients who are looking for relief from sprains and strains
- Voltaren Emulgel Extra Strength Diclofenac Diethylamine 2.32% w/w product details
Voltaren Emulgel Extra Strength Diclofenac Diethylamine 2.32% w/w contains a permeation enhancer
Voltaren contains diclofenac, which is a non-steroidal anti-inflammatory drug (NSAID), that relieves inflammation at the site of pain.1
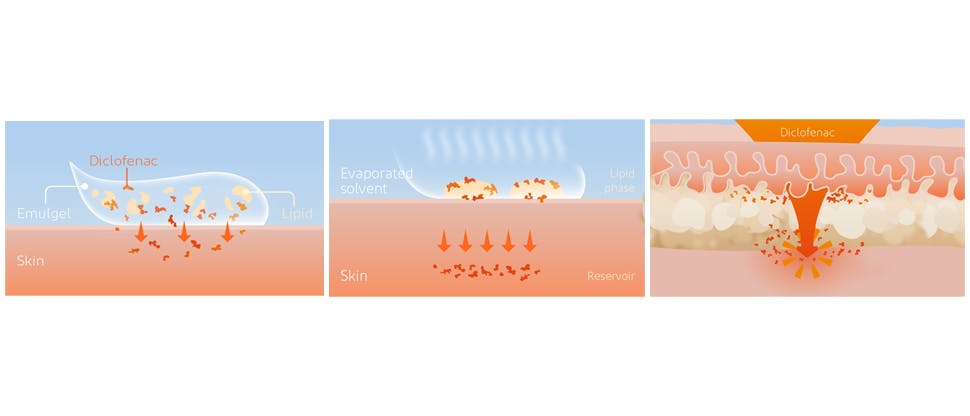
Voltaren Emulgel Extra Strength, containing 2.32% w/w Diclofenac Diethylamine, contains a permeation enhancer Oleyl alcohol2, which belongs to the family of fatty alcohols. Oleyl alcohol is an emulsion stabilizer and acts as a transdermal permeation enhancer, increasing the penetration of diclofenac through the skin.3
A formulation designed for absorption1,4
After topical application, diclofenac is absorbed through the subdermis, and a reservoir is formed in the subdermis, from where it reaches the area of inflammation. Diclofenac persists in the muscle and other tissues, which are the site of pain and inflammation.1,4
When it comes to topical NSAIDs, formulation can be more important than concentration when it comes to absorption:
Results of an in vitro study published by Pradal J, et al. in the Journal of Pain Research (2019), showed that physiochemical properties, such as viscosity, salt, molecular weight and excipients, rather than a higher concentration of active ingredient lead to greater absorption.5
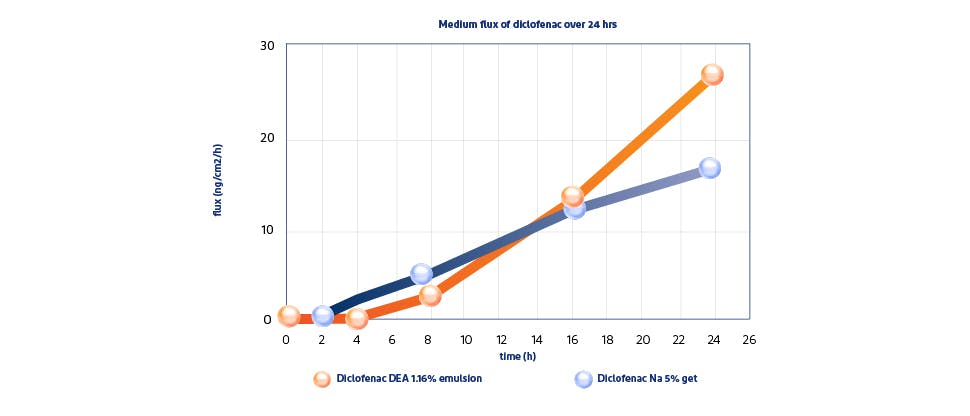
Voltaren Diclofenac Diethylamine 1.16% w/w demonstrated a statistically significant greater permeation through the skin* (Pradal, et al., 2019) at 24 hours vs. diclofenac sodium 5% gel (554 vs. 361 ng/cm2, respectively).5
*Absolute cumulative penetration (ng/cm2) measured over 24 hours. In vitro study on ex vivo skin.
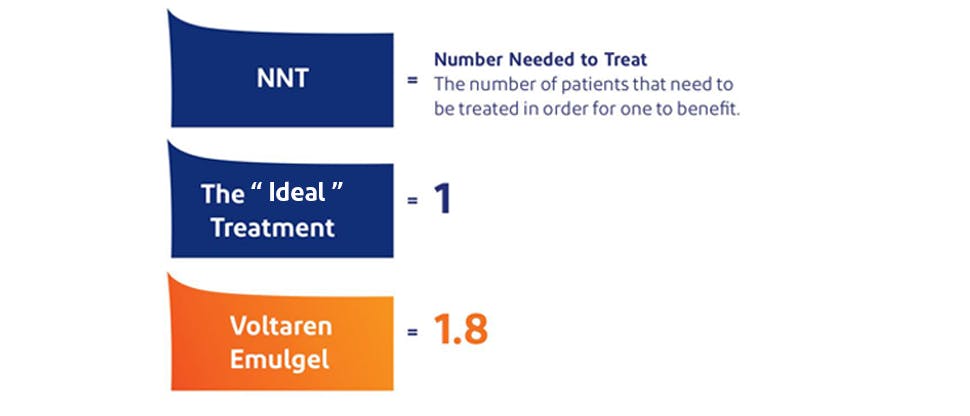
Diclofenac Diethylamine demonstrated a low NNT value, 1.8, for acute musculoskeletal pain6,7
Cochrane reviews are systematic reviews of primary research in human health care and health policy, and are internationally recognized as the highest standard in evidence-based health care.8
In 2015, a Cochrane Review† of 61 studies (>8000 patients) comparing topical NSAIDs in acute musculoskeletal pain reported that Diclofenac Diethylamine demonstrated an NNT value of 1.8.*‡ In comparison, the “ideal treatment” would have an NNT of 1.0.6,7¶
NNT (number needed to treat) is a measure of clinical efficacy and indicates the number of patients that need to be treated in order for one to benefit.9‡
The authors concluded that topical NSAIDs provided good levels of pain relief in acute conditions such as sprains, strains and overuse injuries, probably similar to that provided by oral NSAIDs (Derry, et al., 2015).6
†The review included randomized, double-blind, active or placebo-controlled trials in which treatments were administered to adults with acute pain resulting from strains, sprains or sports or overuse-type injuries.*1.16 % w/w and 2.32 % w/w Voltaren Emulgel formulations included in the review.¶ Reflects a hypothetical scenario where all the patients in the treatment group have benefitted but no one has in the control arm.‡Substantial clinical benefit with Voltaren defined as at least 50% pain reduction.
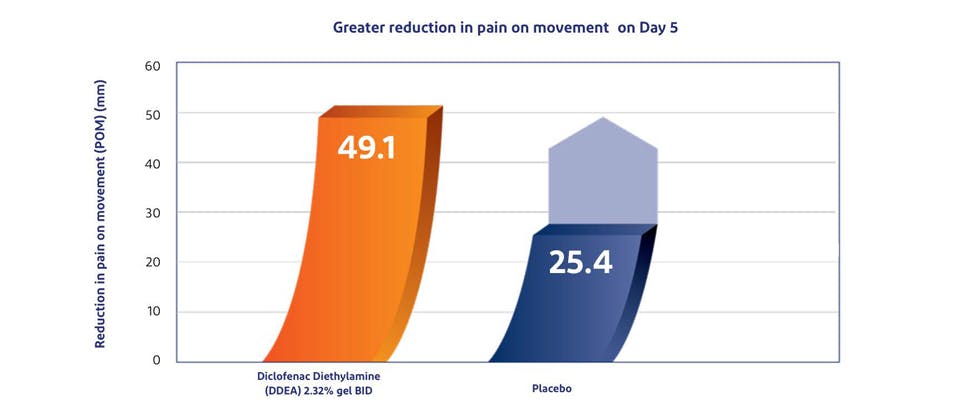
Voltaren provides clinically proven pain relief and greater reduction in pain on movement vs. placebo1,10
In a clinical study by Predel, et al. published in Medicine & Science in Sports & Exercise (2012), patients with acute ankle sprain experienced a 50% or greater reduction in pain-on-movement (POM) with Voltaren Emulgel 2.32% w/w vs. placebo.1,10*†
236 patients completed the trial and were randomized to one of 3 groups, diclofenac diethylamine (DDEA) 2.32% bid, DDEA 2.32% tid and placebo.
The study observed that the onset of efficacy was rapid with both bid and tid DDEA 2.32% treatment regimens and improvements in POM were sustained during the entire 7-day treatment period. By day 5 (the primary efficacy variable), the decrease in POM with DDEA 2.32% bid and tid was twice that observed with placebo (p<0.0001). The difference between the DDEA 2.32% bid and tid groups was insignificant.10
When applied twice daily, Voltaren Emulgel Extra Strength 2.32% w/w provided an earlier reduction in POM vs. placebo 4-5 days sooner in patients with uncomplicated acute ankle sprain.10
*Over day 1-5 (p<0.0001).†Voltaren 2.32% or placebo was applied 2-3 times daily for the duration of the clinical trial. The primary efficacy outcome, ankle POM, was assessed by VAS on day 5. POM on VAS was registered by the patient lying down while the investigator, holding the injured ankle leg at 45̊, performed a gentle supination of the injured ankle to reach an angle of approx. 30̊. Secondary efficacy variables (ankle swelling) were assessed on days 3, 5, and 8.
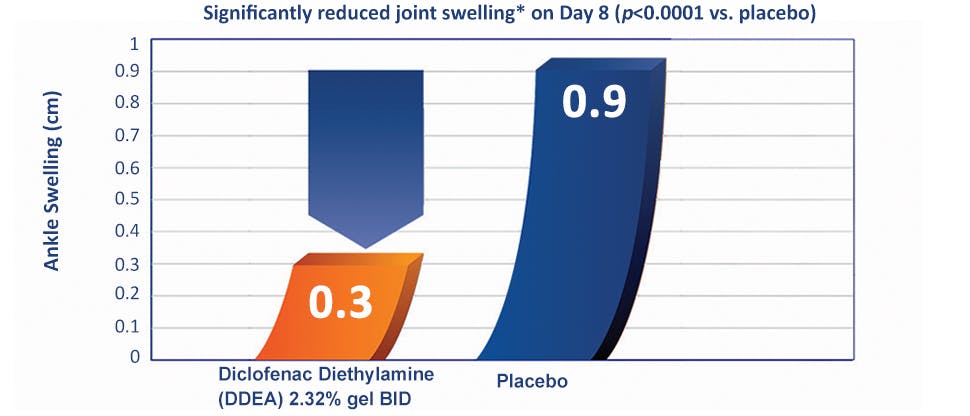
Voltaren provides clinically proven reduction in ankle swelling vs. placebo1,10
In the same clinical study by Predel, et al. published in Medicine & Science in Sports & Exercise (2012), ankle swelling in the Diclofenac Diethylamine 2.32% w/w group, bid & tid, was decreased to one-third of that observed in the placebo group.1,10*†
At all time points, there was a significantly greater decrease in the mean swelling in the groups treated with DDEA 2.32% compared with placebo (p<0.0001), apart from DDEA 2.32% bid at day 3, p=0.0012. The mean change from baseline in the DDEA 2.32% groups was superior to placebo by roughly 0.4 cm at day 3, increasing to roughly 0.6 cm on days 5 and 8. By day 8, the swelling in the DDEA 2.32% bid and tid groups (0.3 cm in both groups) was one third that observed in the placebo group (0.9 cm) p<0.0001.10
*Average reduction in joint swelling with Voltaren at Day 8 vs. averge baseline value (p<0.0001 vs. placebo).
†Voltaren 2.32% or placebo was applied 2-3 times daily for the duration of the clinical trial. Secondary efficacy variables (including ankle swelling) were assessed on days 3, 5, and 8. Ankle swelling was determined by the investigator using the circumference measurement of swelling by the ‘‘figure-of-eight method’’ and subtracting the corresponding measurement of the healthy uninjured ankle.
Voltaren offers low systemic absorption
The low systemic absorption of Voltaren (6% systemic absorption), is associated with a low incidence of systemic side effects.1,4,11
For the full list of side effects, please click here for Voltaren (diclofenac diethylamine 1.16% w/w) or here for Voltaren Emulgel Extra Strength (diclofenac diethylamine 2.32% w/w).
Medication recommendations
To improve pain, inflammation and recovery after acute injuries such as sprains and strains, the National Institute for Health and Care Exellence has developed a clinical knowledge summary for mild-moderate pain, recommending acetaminophen, or a nonsteroidal anti-inflammatory drug (NSAID), oral or topical, as treatment.12

Terry wants effective pain relief for his acute joint injury
Terry balances the demands of his full-time job with taking care of two young children.
He enjoys going to the gym or exercising outdoors and loves nothing more than playing with his children.
The last thing he needs is pain from a sprain that happened while playing tennis affecting his life.
His pain: joint pain
He describes: a red, swollen left ankle; the pain is a little worse after walking
Voltaren is absorbed through the skin to provide relief from pain and inflammation after soft-tissue injuries, so Terry can get back to doing the activities he enjoys.1,10
*Fictional case study.
Voltaren Emulgel Extra Strength Diclofenac Diethylamine 2.32% w/w
Contains a permeation enhancer to increase penetration of diclofenac through the skin.1,2
For patients looking for the relief of pain associated with acute, localized muscle or joint injuries such as sprains, strains or sports injuries.1
Voltaren – for effective relief of acute muscle, back & joint pain1

Voltaren Diclofenac Diethylamine 1.16% w/w
Access information about Voltaren Diclofenac Diethylamine 1.16% w/w variant.

Musculoskeletal pain
Learn more about strains and sprains and how they can be relieved.