Otrivine Extra Dual Relief Nasal Spray
Recommend Otrivine Extra Dual Relief – helps unblock the nose up to 6 times faster than decongestant pills.*1
Unique formulation for a blocked and runny nose from colds.2
- Pharmacy-only medicine
- Nasal congestion, runny nose and sinus pressure are treated simultaneously
- Only product to contain a combination of xylometazoline hydrochloride and ipratropium bromide2
Otrivine Extra Dual Relief contains two active ingredients which work to relieve different nasal symptoms.
Xylometazoline hydrochloride (0.5 mg/ml)
- Reduces the diameter of blood vessels in the nose to reduce nasal mucosal swelling
- Has a decongestant effect
- Fast effect usually obtained after 5-10 minutes and lasts for 6-8 hours
Ipratropium bromide (0.6 mg/ml)
- Reduces nasal mucus secretion
- Fast effect usually obtained within 15 minutes and lasts for an average of 6 hours
Recommend Otrivine Extra Dual Relief to relieve nasal congestion faster than decongestant tablets.*1
*vs pseudoephedrine and phenylephrine tablets.
Download additional resources for your pharmacy

Download our nasal spray guide
Download our free resource to guide your patients on how to use Otrivine Extra Dual Relief Nasal Spray correctly.

Cold and flu consultation pathway
Download this helpful guide for pharmacy staff to help support your conversations with patients about their cold and flu symptoms.
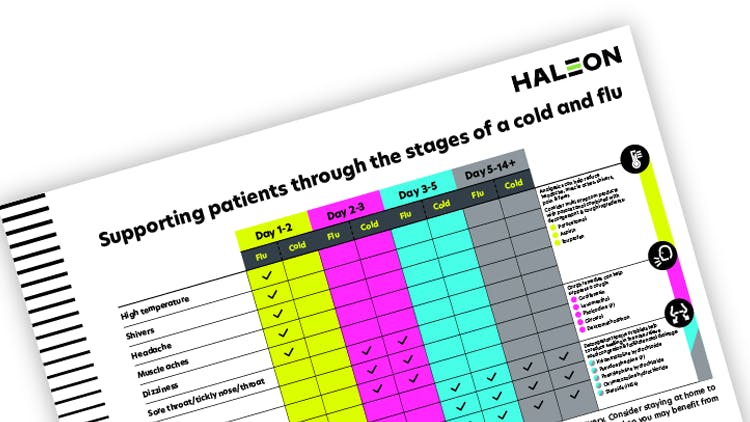
Supporting patients through the stages of a cold and flu
This handy reference sheet outlines the symptoms of a cold & flu over time and includes appropriate ingredients to tackle symptoms.
About Otrivine Extra Dual Relief
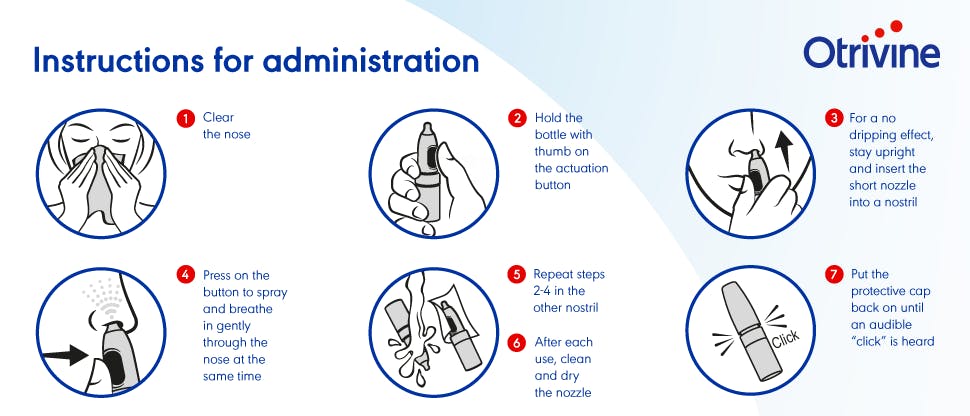
Instructions for use
- One puff in each nostril up to 3 times per day
- Leave at least 6 hours between doses
- Not more than 3 applications daily into each nostril
- The product should not be used for more than 7 days
- Always blow your nose before using the nasal spray
Suitable for use from 18 years and older.
Product Information
Otrivine Extra Dual Relief 0.5 mg/ml + 0.6 mg/ml nasal spray, solution (xylometazoline hydrochloride, ipratropium bromide). Indications: Symptomatic treatment of nasal congestion and rhinorrhea in connection with common colds. Dosage: Adults: 1 puff in each nostril, up to 3 times daily, with at least 6 hours between doses. Maximum 3 applications daily into each nostril. Do not exceed the stated dose. Do not use for longer than 7 days. Geriatrics: Limited experience of use in patients above 70 years of age. Contraindications: Children and adolescents under 18 years. Known hypersensitivity to xylometazoline hydrochloride, ipratropium bromide, any of the excipients, atropine or atropine-like substances. Patients with glaucoma, rhinitis sicca, atrophic rhinitis or following trans-sphenoidal hypophysectomy or surgery exposing the dura mater. Warnings and precautions: Use with caution in people with: hypertension, cardiovascular disease, long QT syndrome, hyperthyroidism, diabetes mellitus, prostatic hypertrophy, bladder stenosis, pheochromocytoma, on monoamine oxidase inhibitors treatment or tri/tetra cyclic antidepressants (or have been on either within the last two weeks), on beta 2-agonist treatment, cystic fibrosis. Use with caution in patients predisposed to angle closure glaucoma, epistaxis or paralytic ileus. Immediate hypersensitivity including urticaria, angioedema and rash may occur. Caution in people sensitive to adrenergic substances. Avoid contact with eyes. Side effects: See SPC for full details. Very common: epistaxis. Common: dysgeusia, rhinalgia, nasal congestion, headache, nasal dryness, nasal discomfort, nausea, application site burn, dizziness, throat irritation, dry throat, dry mouth. Uncommon: insomnia, parosmia, tremor, eye irritation, dry eye, palpitations, tachycardia, nasal ulcer, sneezing, dyspepsia, fatigue, corneal oedema, cough. Price: £6.66 (ex. VAT) Legal Status: P. Licence Number: PL 15545/0002. Licence Holder: GlaxoSmithKline Dungarvan Limited, Knockbrack, Dungarvan, County Waterford, Ireland. Date of revision: October 2023
Recommend Otrivine Extra Dual Relief – Unique formulation for a blocked and runny nose from colds2

The Otrivine range
Find out how the Otrivine range can help your customers and patients.
Recommend Otrivine Extra Dual Relief - Unique formulation for a blocked and runny nose from colds
Educational resources
Access a range of educational resources to support your discussion with patients.

