Otrivine with fine nasal mist*

Otrivine with fine nasal mist*
Otrivine products with the fine nasal mist nozzle offer the same trusted congestion relief as the previous device but with a better patient experience.
Last year, almost 70% of people suffered from nasal symptoms.1 Yet, in many countries, less than 1 in 2 people used nasal sprays to treat their nasal congestion.1
*nozzle provides fine mist
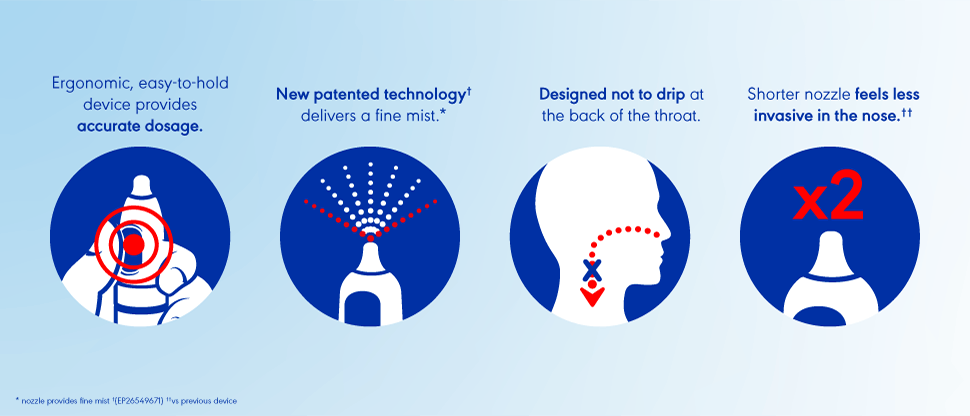
Otrivine with fine nasal mist benefits and product features
Otrivine with fine nasal mist* features patented† technology for comfortable congestion treatment.2
Product features and benefits include:
- An ergonomic design meaning it’s easier** to hold.2
- A gentle mist that reaches wider** to inflamed areas in the nose.3
- Designed not to drip in the back of the throat.3
- A 2x shorter nozzle length, ensuring greater comfort in the nose vs the previous device.2
*nozzle provides fine mist**vs. previous device†Patent number EP2654967B1
Recommend Otrivine with fine nasal mist

Otrivine Fine Nasal Mist*
Otrivine with fine nasal mist* has a patented† technology for comfortable congestion treatment.2-4
*nozzle provides fine mist†EP2654967B1
Otrivine – your Nasal Health Partner

Patient profiles
Learn more about the patients who may benefit from using Otrivine with fine nasal mist* in our patient profiles page.
*nozzle provides fine mist

Educational resources
Access videos and interactive content for pharmacy on respiratory health.