Otri-allergy Nasal Spray
Blocks 6 key inflammatory mediators*13-16
Otri-allergy Nasal Allergy works to help block allergic reactions, in order to relieve allergy symptoms through interference with more than 6 key inflammatory substances, including histamine, prostaglandins, cytokines, tryptases, chemokines and leukotrienes.*13-16
*Mechanism vs most allergy pills. Otri-Allergy acts on multiple inflammatory mediators (histamine, prostaglandins, cytokines, tryptases, chemokines, and leukotrienes). The exact number and precise mechanism are unknown.1

Very reliable profile with few contraindications9,17,18
Otri-allergy Relief is NOT associated with:
- Higher blood pressure, and is not contraindicated in patients with hypertension, heart disease, diabetes, liver disease, or kidney disease8,9,17-20
- Anticholinergic side effects commonly associated with some other allergy medications, such as drowsiness, dry mouth, nervousness, dizziness, or sleeplessness8,9,17-24

Otri-allergy Nasal Spray
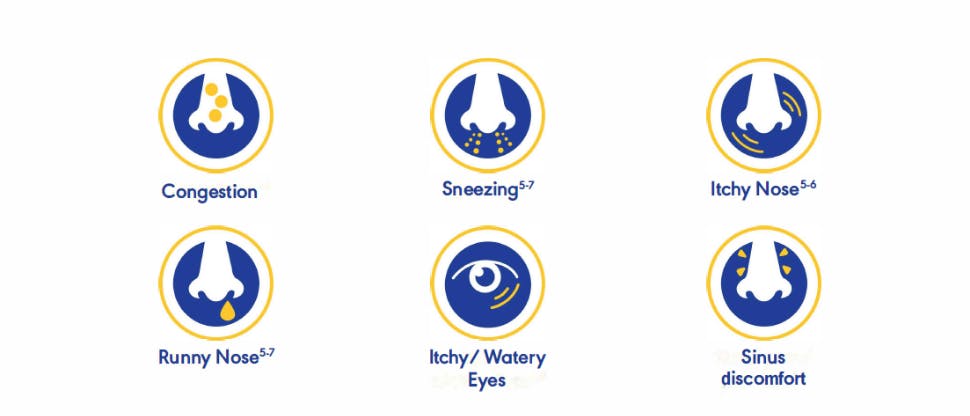
For patients who are looking for 24-hour relief of nasal and ocular symptoms of allergy.1-12

